About ABV-1504 for Clinical Depression (MDD)
Product Overview
The World Health Organization has reported that more than 300 million people worldwide suffer from major depressive disorder (MDD).
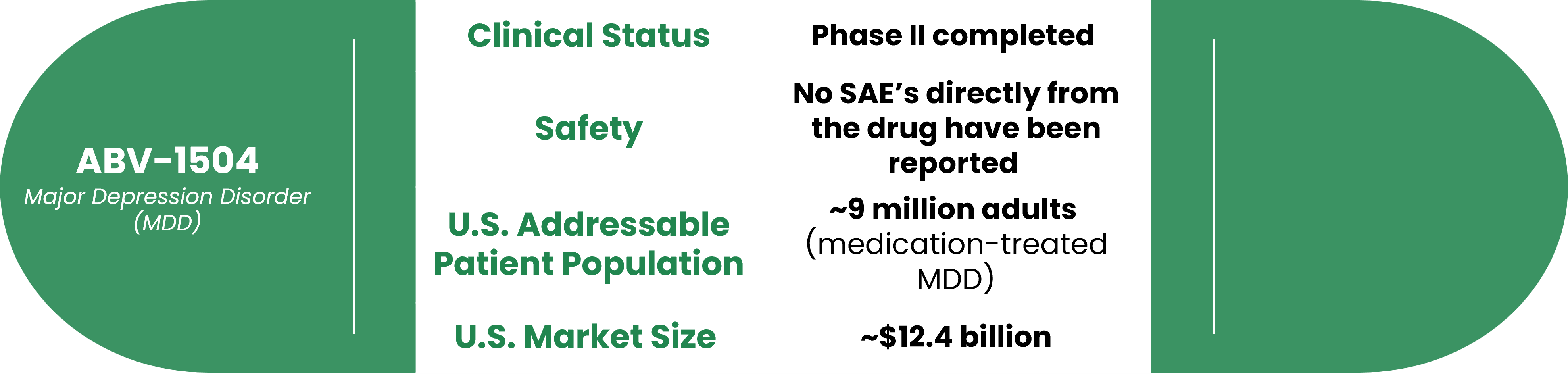
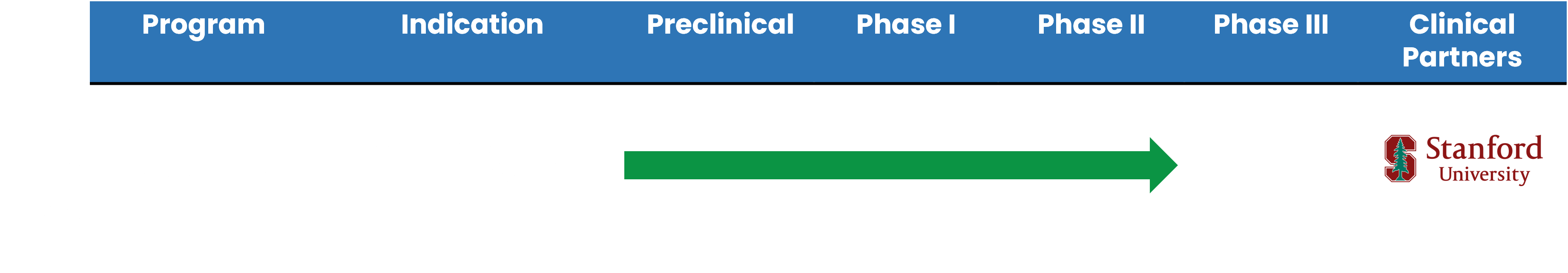
While the market for MDD treatment is very competitive, our solution has strong potential to disrupt the market. We have completed Phase II studies of our plant-based drug and found no adverse side effects. Our studies were conducted by Stanford University and five major medical centers in Taiwan.
ABVC is developing a suite of botanical-based assets to combat rising addiction. We are exploring how to complete Phase III studies for an eventual launch to the market.
Innovative Botanical Asset for MDD
MDD Development Timeline

DISCLAIMER: Clinical trials may be in early stages. There is no guarantee that any specific outcome will be achieved. Investments may be speculative, illiquid and there is a risk of loss. Past performance is not indicative of future results.
