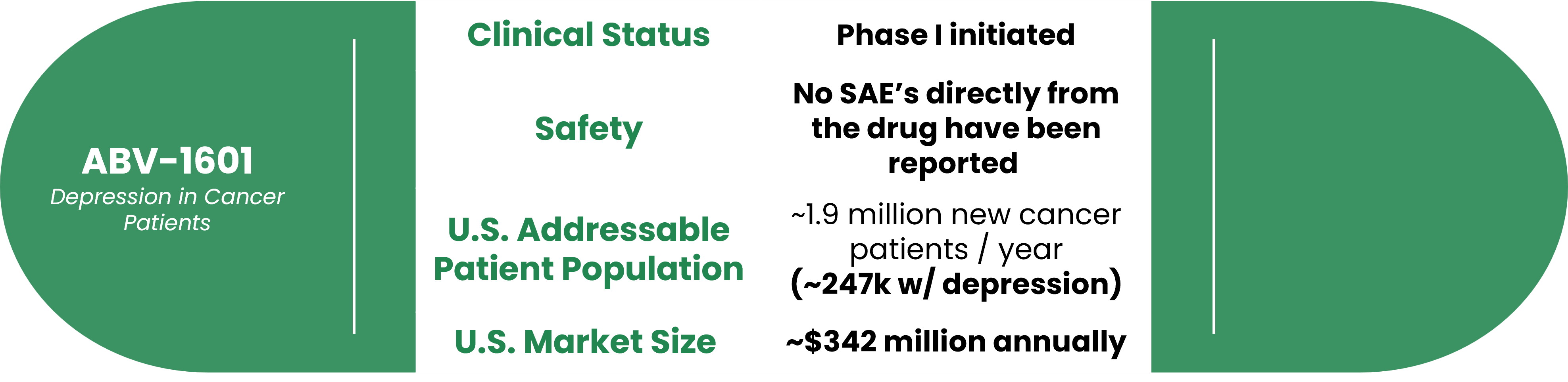
About ABV-1601 for MDD in Cancer Patients
Product Overview
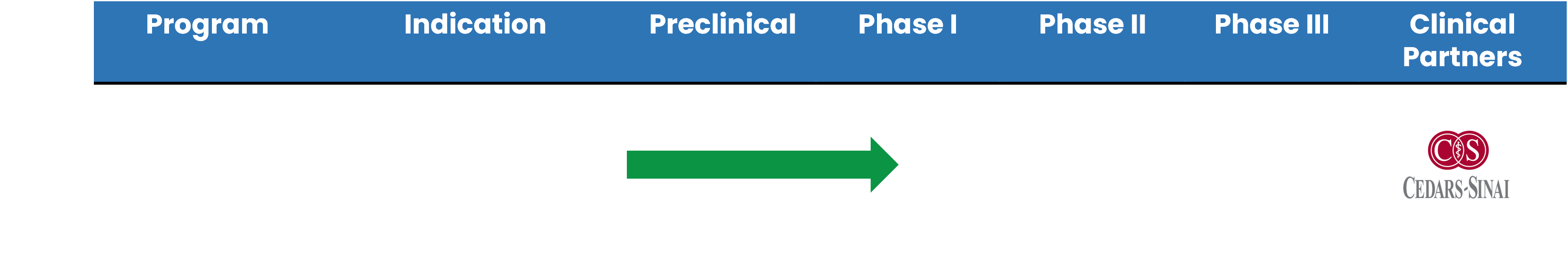
ABV-1601 leverages the same active pharmaceutical ingredient (API) and mechanism of action as ABV-1504 and ABV-1505. The Phase I/II clinical trial for treating depression in cancer patients was approved by the U.S. FDA in December 2018. The Phase I clinical study, led by Scott Irwin M.D., Ph.D. of Cedar-Sinai Medical Center, was initiated in March of 2023.
Botanical Solutions for Cancer Patients
ABV-1601 Development Timeline

DISCLAIMER: Clinical trials may be in early stages. There is no guarantee that any specific outcome will be achieved. Investments may be speculative, illiquid and there is a risk of loss. Past performance is not indicative of future results.
