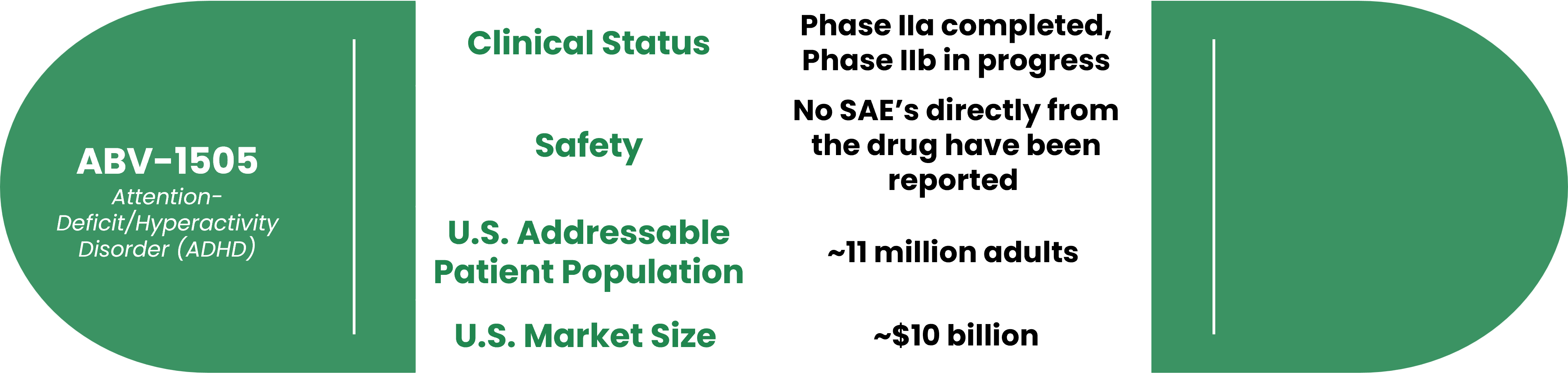
About ABV-1505 for ADHD
Product Overview
Did you know that only 11% of adults with ADHD are being treated for it? As mental health awareness continues to grow in the United States, an increasing number of individuals are seeking safer alternatives to treat ADHD.
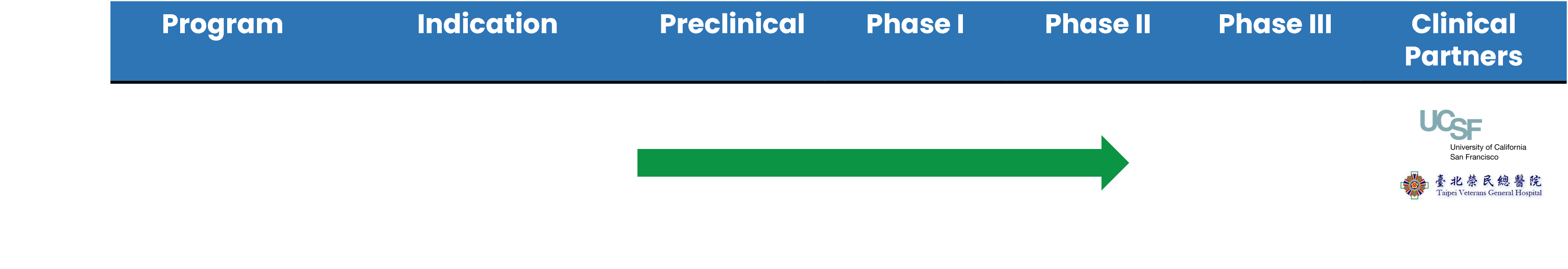
This market, which is expected to reach US$24.9 billion in the next two years according to Grand View Research, needs safe treatment options. Our botanical drug is based on traditional Chinese Medicine and has no adverse effects or addiction in Phase I and Phase II Part 1 clinical studies. 5 out of 6 subjects showed a 40% improvement in our trials conducted at UCSF Medical Center. Phase II Part 2 clinical study is currently conducted at UCSF Medical Center and five major hospitals in Taiwan which is expected to complete by the end of 2023.
Innovative Botanical Asset for ADHD
ADHD ABV-1505 Development Timeline

DISCLAIMER: Clinical trials may be in early stages. There is no guarantee that any specific outcome will be achieved. Investments may be speculative, illiquid and there is a risk of loss. Past performance is not indicative of future results.
