About Vitargus® for Vitreoretinal Vitrectomy
Product Overview
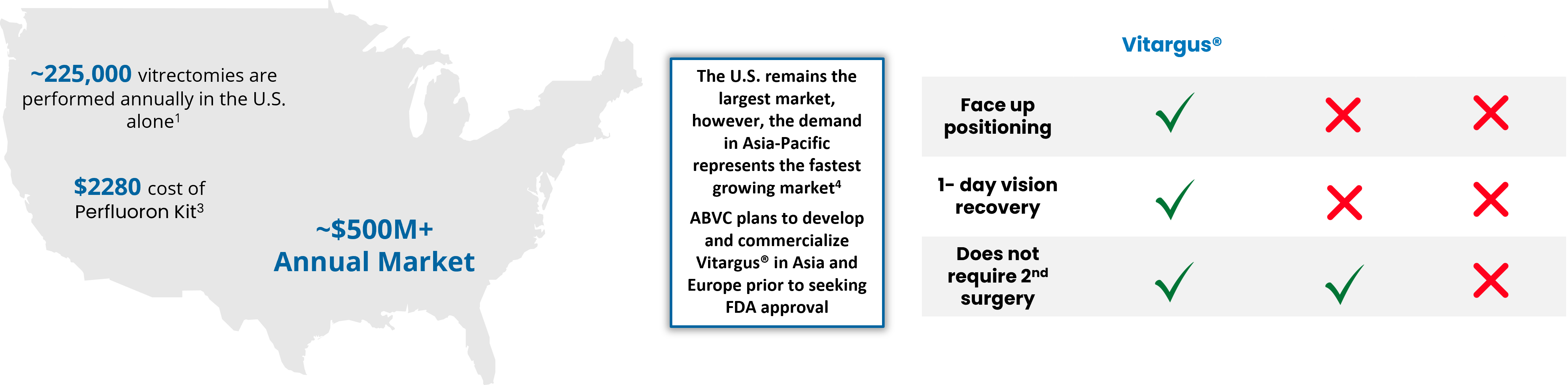
The Global Retinal Detachment Disorder Market Size was valued at USD 1.7 billion in 2021 with millions of patients receiving eye surgery for retinal detachment and vitreous hemorrhage using current vitreous substitutes according to a research report published by Spherical Insights & Consulting.
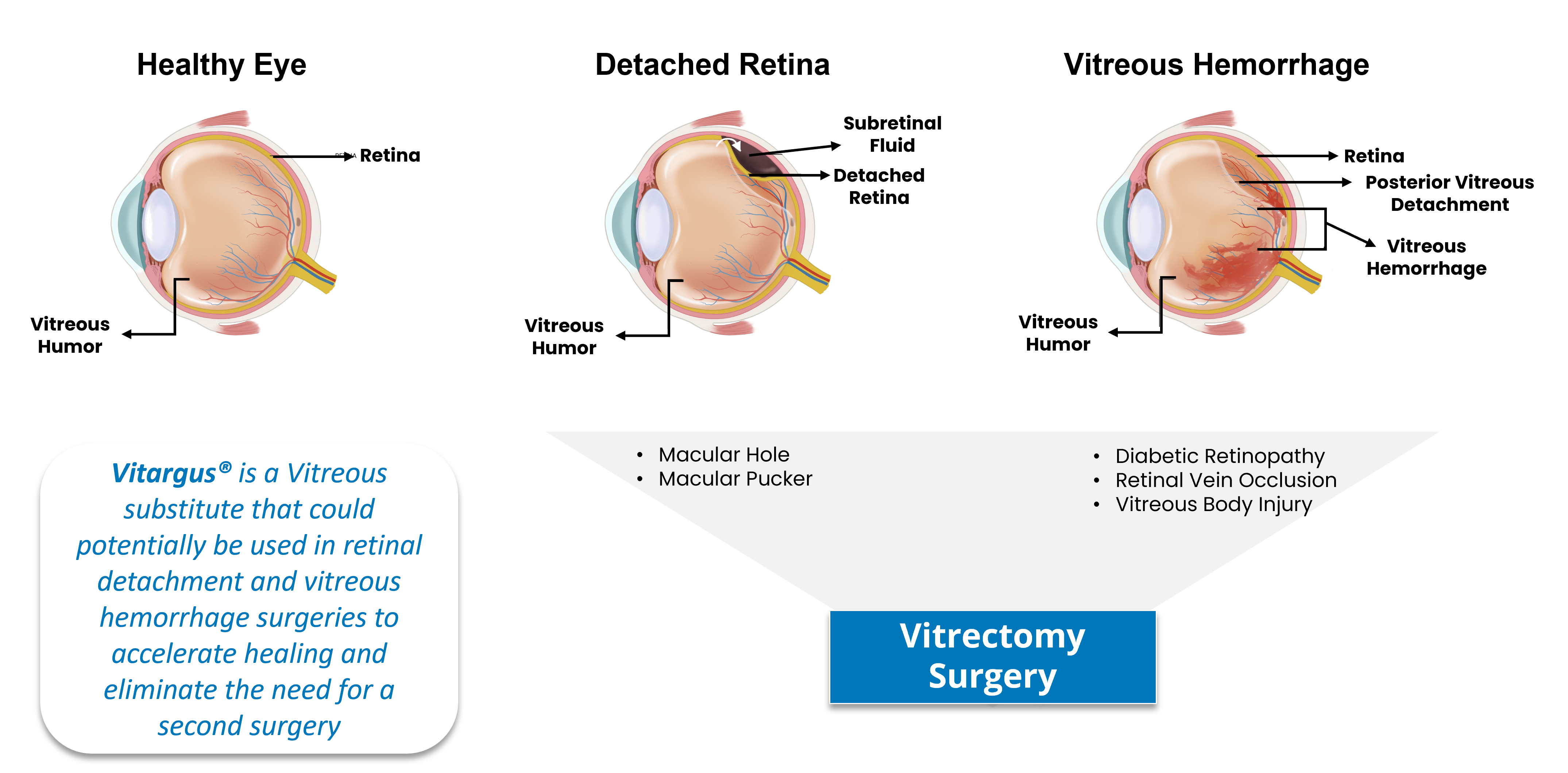
Vitargus is the first biodegradable vitreous substitute to facilitate retinal reattachment surgery. The optical properties of Vitargus® allowed the patients to see well and facilitated visualization of the fundus immediately following surgery.
ABVC is looking to bring this product to the market in Asia and Europe before seeking FDA approval to bring it stateside.
Vitargus® – Vitrectomy Surgery Assistance
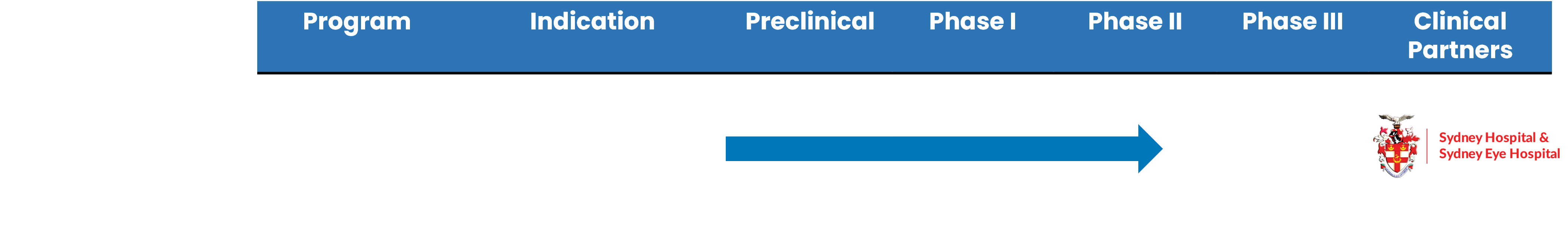
Vitargus® Development Timeline

DISCLAIMER: Clinical trials may be in early stages. There is no guarantee that any specific outcome will be achieved. Investments may be speculative, illiquid and there is a risk of loss. Past performance is not indicative of future results.
