ABVC’s Business Model
ABVC identifies and in-licenses compounds from our network of world-class research institutions. Drugs considered by ABVC are typically derived from plants, address significant illness and unmet needs, and have successfully completed pre-clinical, disease animal model studies, and/or Phase 1 safety studies at world-renowned research institutions. Upon successful completion of the Phase 2 trial, ABVC seeks a partner – typically a large, global pharmaceutical company – to complete Phase 3, submit the New Drug Application (NDA), and commercialize the asset upon FDA approval.”
New Drug Development

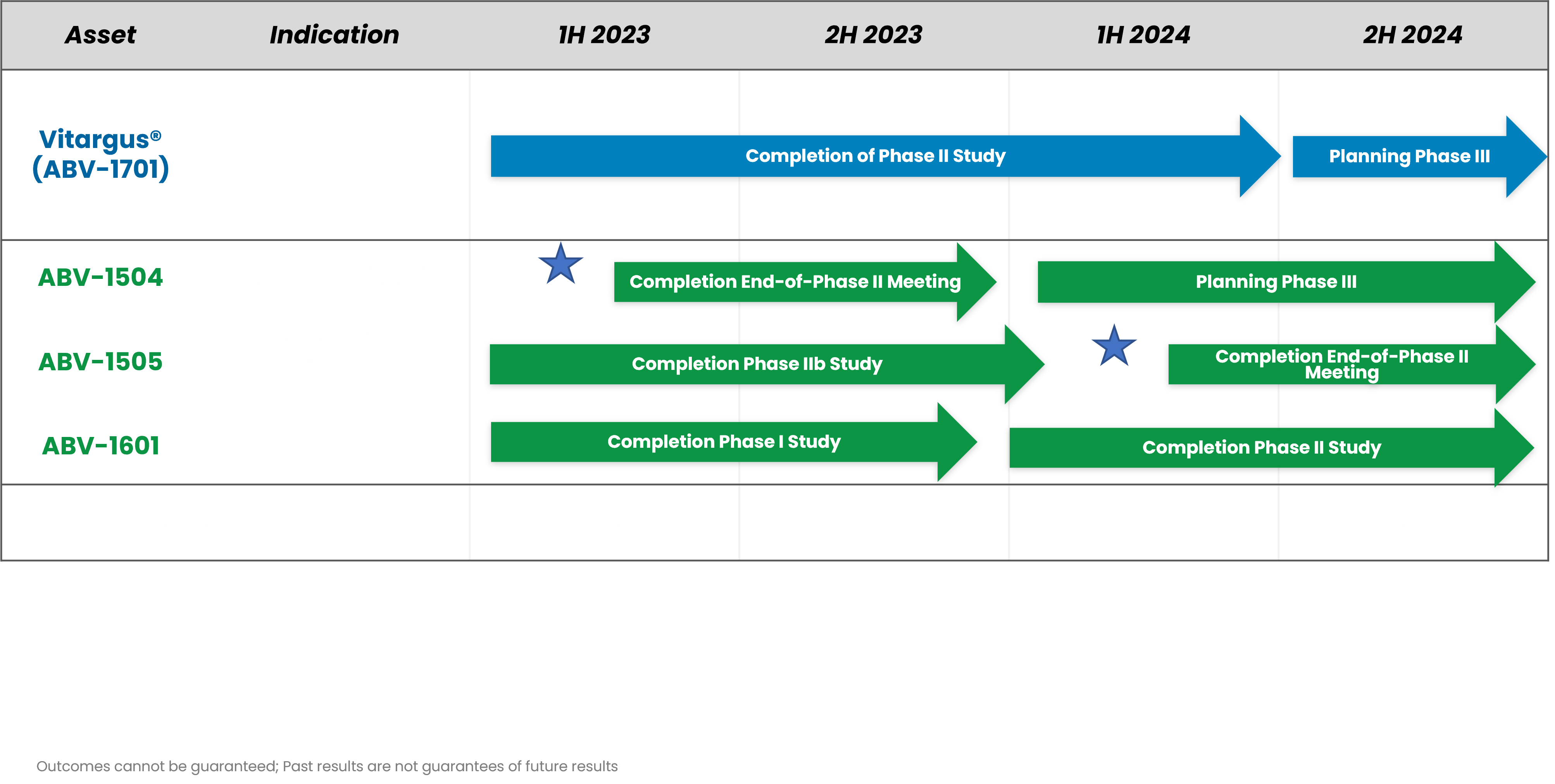
Product Development Timeline